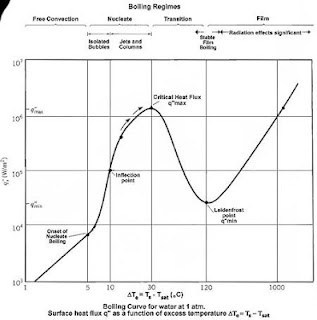
Boiling is a great means of heath transfer. Though it is a very much familiar form of heat transfer, its mechanism is still very confusing. The most important inventions in the field of boiling was done by S. Nukiama in 1934.
Different boiling regimes
Four boiling regimes are seen in the boiling curve. These are
- Natural convection regime
- Nucleate regime
- Transition regime and
- Film boiling regime
Natural convection boiling
Boiling starts when the fluid come into its saturation temperature. But actually it needs some more temp. to boiling to be started. For water this temperature is about 2 to 6 degrees. At that condition we start to see the bubbles forming. In the natural convection boiling we start to see the bubbles to form. Bubbles are an indispensable part of boiling. No needs telling that in this region all the heat transfer is by natural convection currents. This stage happens when the fluid is slightly superheated (metastable).
Nucleate boiling
This is the most desirable part of boiling heat transfer. In
this part we can see the regions. At this part bubbles start to form in
different nucleation sites. These bubbles are formed and in the first region
they are most likely to collapse when they leave the heater surface. These are
isolated bubbles. There is a significant increase in the rate of bubble
formation. When bubbles leave the surface they collapse and the space vacated
is filled by the surrounding liquid and thus heat transfer is accelerated.
After taking a lot of heat the bubbles are big enough and
they rise to the top surface. These bubbles are energy movers. Thus we can see
a significant increase in the boiling curve with the excess temperature. In
this region they form continuous columns of bubbles. Here we find the highest
heat flux or critical heat flux or the burning heat flux. This point is also
called the burning point or critical heat flux.
Transition boiling
It is a very much undesired and unstable part of boiling. In
this stage we can see a huge drop of heat flux. The reason behind this is the
vapor blanket. Liquids take too much heat and they form huge amount of vapor
that cause the formation of vapor blanket or vapor cover. Thus the surrounding liquids
find it hard to get into the heater surface. That’s why we see the significant heat
flux drop. Here the heat transfer rate decreases.
Film boiling
Here is the interesting part. Here we see that heat transfer
increases. After getting the critical heat flux and then a decrease in the
transition boiling the heater surface absorbs heat. And after sometimes it
leaves the heat into the liquid and the heat is very massive that radiation
heat transfer comes in handy. That’s why we see an increase in the heat flux. At
the first part part of this region we see a minimum flux and that is called Leidenfrost
point or the minimum heat flux point. In this part the burnout phenomenon
can occur very frequently.





Helpful Material
ReplyDeleteSimple and Good
ReplyDeletenice
ReplyDeleteachha hai
ReplyDeletegreat
ReplyDeleteNice infiinforma
ReplyDeleteReally helpful thank you
ReplyDeleteThanks a lot
ReplyDeleteToo much helpful.. Thanks
ReplyDelete